Mitochondrial Function and Brain Longevity
Part I: More than just “powerhouses” - how Mitochondria Shape Brain Health and Longevity
Summary
Mitochondria are far more than mere energy factories — they are dynamic regulators of oxidative stress, neuroinflammation, signaling, cell survival, and epigenetic expression.
Understanding how mitochondria influence brain health and longevity and how these processes can become dysfunctional reveals the cellular roots of neurodegeneration and other brain disorders and offers new therapeutic avenues to preserve cognitive function and mental vitality across the lifespan.
Mitochondrial Function: The Core of Brain Health and Longevity
Longevity has become the big theme in modern health - and it is here to stay. Who doesn’t want to live long and stay healthy, vibrant, and mentally sharp while doing so?
Among all aspects of longevity, maintaining brain health may be the greatest challenge. Just think about how dramatically the incidence of degenerative brain disorders such as the different types of dementia or Parkinson’s Disease rises. Despite decades of research, we still lack therapeutic strategies that truly address the underlying mechanisms driving these conditions.
I belong to a growing group of scientists who believe that many answers and new strategies can be found when we turn our attention to the contributions of mitochondria to brain health resp. disease. For decades, they’ve been described merely as the powerhouses of our cells. But recent science reveals a far richer story: mitochondria are not just energy factories; they are master regulators of cellular health, resilience, and signaling — especially in the brain.
1. Mitochondria as Energy Providers
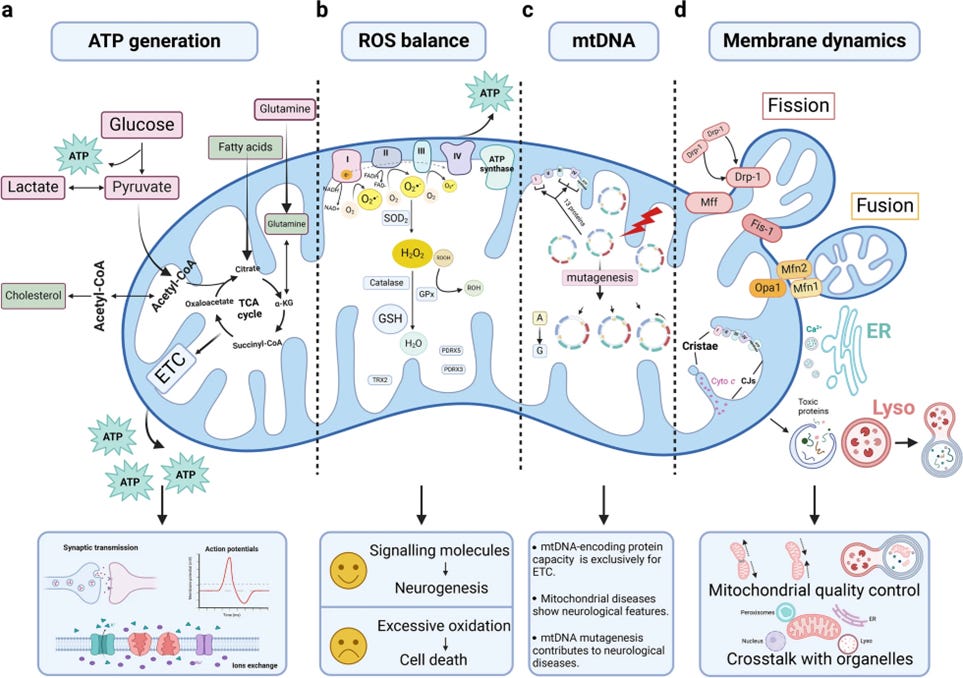
We all learned in biology class that the trillions of mitochondria generate adenosine triphosphate (ATP), the universal energy currency that powers nearly every cellular process. In the brain, ATP fuels the synthesis, release, and reuptake of neurotransmitters; regulates postsynaptic calcium (Ca²⁺) signaling; drives ion pump activity; supports receptor activation; enables axonal transport; and sustains countless other energy-dependent mechanisms essential for neural communication.
A continuous and reliable ATP supply is particularly critical in our energy-hungry brains. Although the brain accounts for only about 2% of total body weight, it consumes roughly 20% of our body’s energy. This means that even slight impairments in mitochondrial energy production can have profound effects on neuronal function — and, by that, on cognition, mood, and overall brain performance.
“The more energetically demanding a cell is, the more mitochondria they have, and the more critical that mitochondria health is — so there’s more potential for things to go wrong.”
* Andrew Moehlman, postdoctoral researcher who studies neurodegeneration at the US National Institute of Neurological Disorders and Stroke (NINDS).
2. Mitochondria and Neuroinflammation
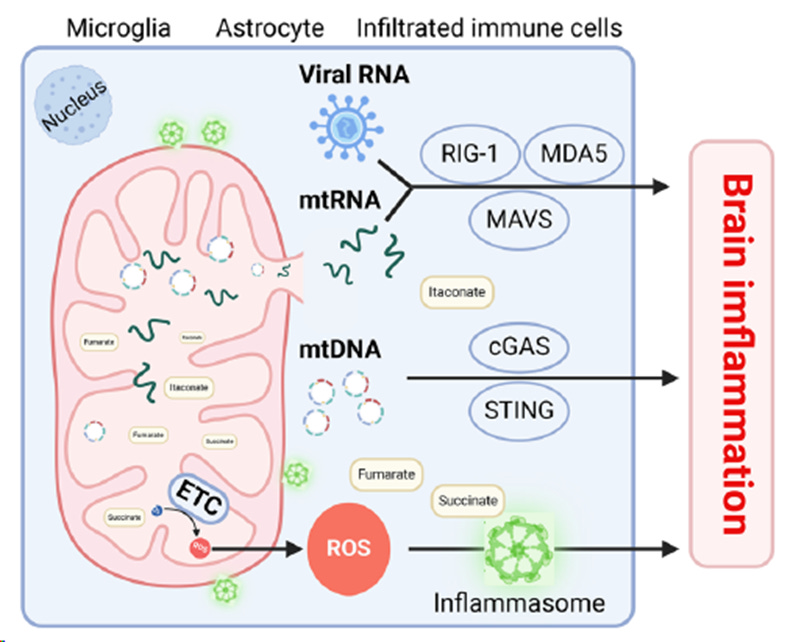
Mitochondria contain several endogenous inflammatory triggers — including mitochondrial DNA (mtDNA), RNA (mtRNA), metabolic by-products, and reactive oxygen species (ROS). Under normal circumstances, these components are efficiently managed and removed by the cell’s “mitochondrial quality control system”, through processes such as mitophagy and other immune-regulating mechanisms.
However, when cells become stressed or damaged, these mitochondrial molecules can escape into the extracellular environment. There, they are mistaken by the immune system as foreign invaders — so-called damage-associated molecular patterns (DAMPs) — and activate pattern recognition receptors (PRRs), leading to the release of pro-inflammatory cytokines.
This can establish a vicious cycle: inflammation further impairs mitochondrial function, while mitochondrial dysfunction perpetuates inflammation. Over time, this self-reinforcing loop contributes to neuronal damage.
A growing body of evidence underscores the intimate link between neuroinflammation and brain health — and, more broadly, identifies inflammation as a fundamental driver of the aging process itself.
Here is an interesting paper if you want to dive deeper into this.
Song, N., Mei, S., Wang, X., Hu, G., & Lu, M. (2024). Focusing on mitochondria in the brain: from biology to therapeutics. Translational neurodegeneration, 13(1), 23. https://doi.org/10.1186/s40035-024-00409-w
3. Mitochondria and Oxidative Stress: Balancing Reactive Oxygen Species for Brain Health
Reactive Oxygen Species (ROS) generation is an inevitable byproduct of mitochondrial respiration. During normal electron transport, electrons flow smoothly through complexes I,II, and II to finally reduce oxygen to water in complex IV (Cytochrome C Oxidase). However, when this flow becomes partially uncoupled or inefficient, electrons can prematurely react with oxygen at complexes I and III, forming superoxide and other reactive oxygen species.
While ROS are not inherently pathological, problems arise when their generation exceeds the cell’s antioxidant capacity, tipping the balance toward oxidative stress.
Besides contributing to neuroinflammation, oxidative stress can cause damage on mitochondrial proteins, lipids, and DNA, thereby compromising both mitochondrial function and replication.
Moreover, ROS can disturb the electron flow within the mitochondrial respiratory chain, reducing the efficiency of ATP synthesis and increasing electron leakage — a process that further amplifies ROS generation in a self-perpetuating cycle.
Excessive oxidative stress can also disrupt the mitochondrial membrane potential, interfering with essential cellular signaling pathways and ultimately undermining neuronal integrity and resilience.
4. Mitochondrial DNA mutations
Mitochria contain their own genetic material, a small circular genome located within the mitochondrial matrix. This mtDNA encodes essential proteins required for oxidative phosphorylation and energy production — making it crucial for normal cellular and neuronal function.
Unlike nuclear DNA, mitochondrial DNA is particularly vulnerable to mutations. Its replication occurs continuously to meet fluctuating cellular energy demands, and its close proximity to the electron transport chain exposes it to high levels of reactive oxygen species (ROS). In addition, mitochondria possess only limited DNA repair capacity, which further increases the risk of oxidative damage and mutagenesis.
Over time, the accumulation of mtDNA mutations can impair mitochondrial protein synthesis and disrupt normal respiratory chain activity. Maintaining the integrity of mitochondrial DNA is therefore a key aspect of cellular and brain longevity.
5. Mitochondria and Membrane Dynamics
One of the remarkable features of mitochondria is their ability to self-replicate through a process known as binary fission, in which a single mitochondrion divides into two smaller organelles. In addition to this, mitochondria are in a constant state of flux, continuously undergoing fusion, fission, and selective degradation (mitophagy).
These dynamic membrane processes allow mitochondria to adapt to changing cellular energy demands, share contents such as proteins and DNA, and remove damaged components. However, when this delicate balance is disturbed, mitochondrial networks can fragment or collapse, compromising energy production and cellular homeostasis.
Through their extensive membrane dynamics and organelle interactions, mitochondria influence — and can disrupt — the function of other key cellular systems, including the endoplasmic reticulum, lysosomes, and peroxisomes, thereby affecting overall neuronal health and signaling integrity.
Mitochondrial dynamics in health and disease: mechanisms and potential targets
Song, N., Mei, S., Wang, X., Hu, G., & Lu, M. (2024). Focusing on mitochondria in the brain: from biology to therapeutics. Translational neurodegeneration, 13(1), 23. https://doi.org/10.1186/s40035-024-00409-w
6. Mitochondrial Calcium Homeostasis
Mitochondria are also central regulators of cellular calcium homeostasis. They act as dynamic calcium buffers, taking up and releasing Ca²⁺ to fine-tune intracellular signaling — processes that are vital for neuronal excitability, neurotransmitter release, and synaptic plasticity.
However, when mitochondrial function is compromised, calcium handling becomes dysregulated. Excessive mitochondrial calcium uptake can trigger the opening of the mitochondrial permeability transition pore, leading to loss of membrane potential, swelling, and ultimately cell death. Conversely, insufficient calcium buffering disrupts synaptic transmission and neuronal communication.
Thus, maintaining balanced mitochondrial calcium flux is essential for preserving neuronal stability and cognitive performance, while calcium overload represents a critical event linking mitochondrial dysfunction to neurodegeneration.
Mitochondrial calcium signalling and neurodegenerative diseases - PMC
7. Mitochondria and Gene Expression: The Epigenetic Connection
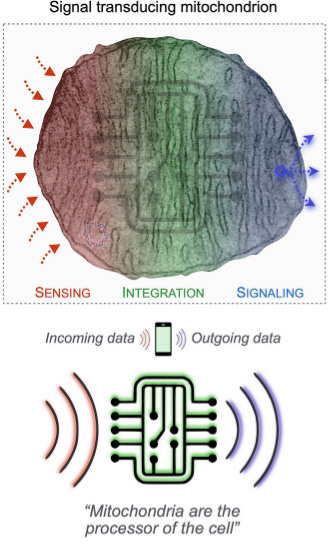
Beyond their metabolic and signaling roles, mitochondria also act as epigenetic regulators, directly linking the status of and around a cell to nuclear gene expression. They continuously sense inputs from the extracellular environment — such as hormones, nutrients, and metabolites — and integrate this information through dynamic remodeling processes, including fusion, fission, organelle interactions, and network interactions. In response, mitochondria transmit signals back to the nucleus in the form of metabolites, proteins, and mitochondrial DNA, thereby modulating gene transcription.
This regulatory action is primarily driven by the fact that many of the substrates and cofactors required by histone- and DNA-modifying enzymes — such as acetyl-CoA, NAD⁺, and α-ketoglutarate — are direct products of mitochondrial metabolism.
This integrative signaling framework has been described as the Mitochondrial Information Processing System (MIPS), emphasizing mitochondria’s role as a central hub in coordinating cellular adaptation and gene regulation.
Mitochondrial signal transduction - PMC
Picard, M., & Shirihai, O. S. (2022). Mitochondrial signal transduction. Cell metabolism, 34(11), 1620–1653. https://doi.org/10.1016/j.cmet.2022.10.008
Mitochondria: The link between Aging, Stress, Lifestyle, and Brain Longevity
Being essential to brain health in countless ways, mitochondria represent finely tuned systems — yet they are also remarkably vulnerable to disruption. Aging, chronic psychological and metabolic stress, environmental toxins, and lifestyle-related factors can all conspire over time to erode mitochondrial efficiency and resilience.
An expanding body of research now identifies mitochondrial dysfunction as a unifying mechanism underlying a wide range of neurodegenerative diseases, including Alzheimer’s, Parkinson’s, and amyotrophic lateral sclerosis. Notably, 30–50% of children with autism exhibit biochemical or genetic signs of impaired mitochondrial function, positioning mitochondria as a potential missing link between neurodevelopmental vulnerability and environmental influences. Emerging “metabolic theories of mental disorders” further suggest that mitochondrial dysfunction contributes not only to neurodegeneration but also to psychiatric conditions such as depression, anxiety, and related mood disorders — reinforcing the idea that mental and metabolic health are deeply intertwined at the cellular level.
The encouraging news is that mitochondrial biology is not fixed — it can be repaired, recalibrated, and strengthened through various interventions. As research continues to uncover the pathways of mitochondrial resilience, it becomes clear that our cells retain a remarkable capacity for renewal.
Part II will explore what we can currently do to support mitochondrial health and thereby enhance cellular energy production, and sustain cognitive vitality throughout the lifespan.
References
Abadin, X., de Dios, C., Zubillaga, M., Ivars, E., Puigròs, M., Marí, M., Morales, A., Vizuete, M., Vitorica, J., Trullas, R., Colell, A., & Roca-Agujetas, V. (2024). Neuroinflammation in Age-Related Neurodegenerative Diseases: Role of Mitochondrial Oxidative Stress. Antioxidants (Basel, Switzerland), 13(12), 1440. https://doi.org/10.3390/antiox13121440
Clemente-Suárez, V. J., Redondo-Flórez, L., Beltrán-Velasco, A. I., Ramos-Campo, D. J., Belinchón-deMiguel, P., Martinez-Guardado, I., Dalamitros, A. A., Yáñez-Sepúlveda, R., Martín-Rodríguez, A., & Tornero-Aguilera, J. F. (2023). Mitochondria and Brain Disease: A Comprehensive Review of Pathological Mechanisms and Therapeutic Opportunities. Biomedicines, 11(9), 2488. https://doi.org/10.3390/biomedicines11092488
Chen, W., Zhao, H. & Li, Y. Mitochondrial dynamics in health and disease: mechanisms and potential targets. Sig Transduct Target Ther 8, 333 (2023). https://doi.org/10.1038/s41392-023-01547-9
Valiente-Pallejà, A., Tortajada, J., Bulduk, B. K., Vilella, E., Garrabou, G., Muntané, G., & Martorell, L. (2022). Comprehensive summary of mitochondrial DNA alterations in the postmortem human brain: A systematic review. EBioMedicine, 76, 103815. https://doi.org/10.1016/j.ebiom.2022.103815
Britti, E., Delaspre, F., Tamarit, J., & Ros, J. (2018). Mitochondrial calcium signalling and neurodegenerative diseases. Neuronal signaling, 2(4), NS20180061. https://doi.org/10.1042/NS20180061
Picard, M., & Shirihai, O. S. (2022). Mitochondrial signal transduction. Cell metabolism, 34(11), 1620–1653. https://doi.org/10.1016/j.cmet.2022.10.008